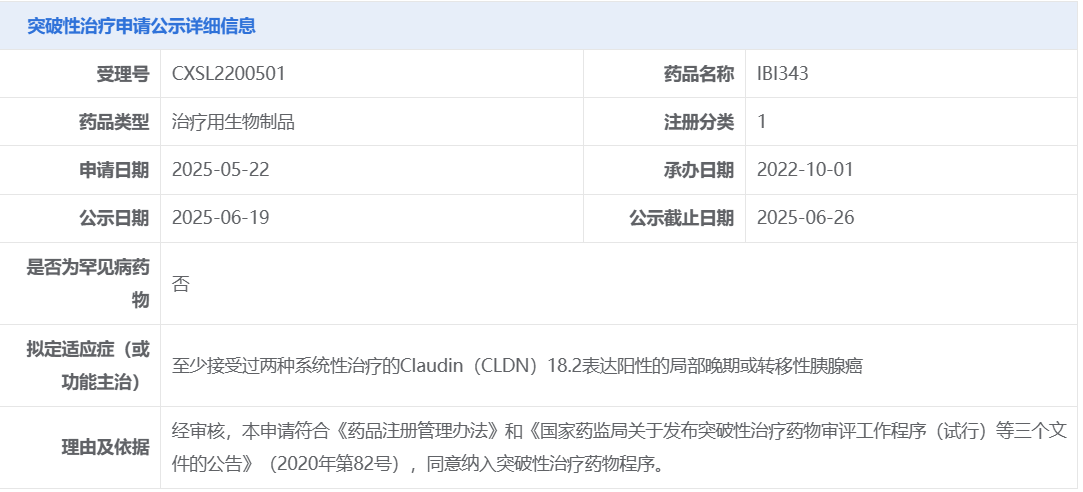
On June 19, the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) announced that Innovent Biologics’ IBI343 has been proposed for inclusion in the breakthrough therapy designation program, intended for the treatment of locally advanced or metastatic pancreatic cancer that is CLDN18.2-positive and has received at least two prior systemic therapies.
IBI343 is a recombinant humanized anti-CLDN18.2 antibody-drug conjugate (ADC). Upon binding to tumor cells expressing CLDN18.2, it undergoes CLDN18.2-dependent ADC internalization and releases a cytotoxic payload, leading to DNA damage and ultimately inducing tumor cell apoptosis.
On December 9, 2024, the latest data from the Phase I clinical trial (NCT05458219) of IBI343 in advanced pancreatic ductal adenocarcinoma (PDAC) were presented at the European Society for Medical Oncology Asia (ESMO Asia) Annual Meeting.
A total of 43 patients with CLDN18.2-positive advanced PDAC were treated with IBI343. The results showed an overall objective response rate (ORR) of 32.6%, a confirmed objective response rate (cORR) of 23.3%, and a confirmed disease control rate (cDCR) of 81.4%. The median duration of response (mDoR) was 7.0 months, with a 6-month DoR rate of 63%, and the median progression-free survival (PFS) was 5.3 months.
At present, there are still many clinical trials of new anti-cancer technologies in China seeking patients. Consultation on new drugs and technologies, you can contact Beijing South Region Oncology Hospital International Department.
Phone Number:4008803716
Email:myimmnet@163.com
Post time: Jun-19-2025

