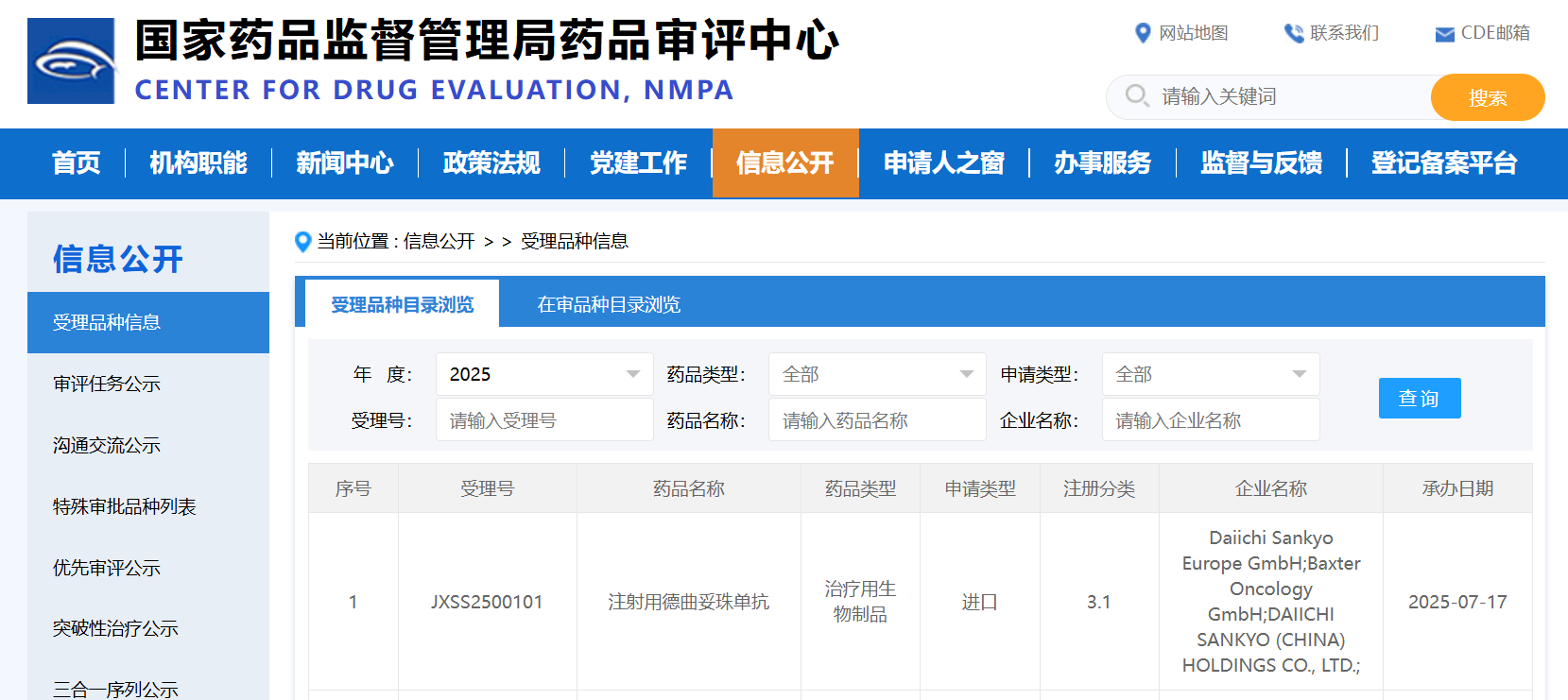
On July 17, the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) announced that the marketing application for Trastuzumab Deruxtecan (Enhertu®) for the second-line treatment of gastric cancer has been officially accepted by the NMPA. This application is intended for the treatment of adult patients with HER2-positive gastric or gastroesophageal junction adenocarcinoma in the second-line setting.
ENHERTU (trastuzumab deruxtecan; fam-trastuzumab deruxtecan-nxki in the U.S. only) is a HER2 directed ADC. Designed using Daiichi Sankyo’s proprietary DXd ADC Technology, ENHERTU is the lead ADC in the oncology portfolio of Daiichi Sankyo and the most advanced program in AstraZeneca’s ADC scientific platform. ENHERTU consists of a HER2 monoclonal antibody attached to a number of topoisomerase I inhibitor payloads (an exatecan derivative, DXd) via tetrapeptide-based cleavable linkers.
At present, there are still many clinical trials of new anti-cancer technologies in China seeking patients. Consultation on new drugs and technologies, you can contact Beijing South Region Oncology Hospital International Department.
Phone Number:4008803716
WeChat ID: 17801183037
Email:myimmnet@163.com
This listing application is mainly based on the statistically significant and clinically significant results of the DESTINY-Gstric04 study (which were presented at the American Society of Clinical Oncology ASCO Conference in 2025).
DESTINY-Gastric04 (NCT04704934),a global, randomized, multicenter, open-label, phase 3 study evaluating the efficacy and safety of T DXd vs RAM + PTX in pts with HER2+ unresectable/metastatic GC/GEJA in this second-line setting.
Methods:
After biopsy-confirmed HER2+ status (IHC 3+ or IHC 2+/ISH+), pts were randomized 1:1 to T-DXd 6.4 mg/kg or RAM + PTX. The primary endpoint was overall survival (OS). OS between the 2 arms was compared by a log-rank test stratified using randomization factors. Secondary endpoints by investigator assessment include progression-free survival (PFS), confirmed objective response rate (cORR), disease control rate (DCR), and safety.
Results:
At data cutoff (October 24, 2024), 494 pts were assigned (T-DXd, n = 246; RAM + PTX, n = 248). Based on 266 OS events observed (information fraction = 78.5%), efficacy superiority was achieved (2-sided P < 0.0228). Median (m) (95% CI) OS follow-up was 16.8 mo (14.0-20.0) for T-DXd and 14.4 mo (13.1-19.7) for RAM + PTX. mOS (95% CI) was 14.7 mo (12.1-16.6) for T-DXd vs 11.4 mo (9.9-15.5) for RAM + PTX (hazard ratio [HR], 0.70; P = 0.0044). Additional efficacy data are in the Table. Median (range) treatment duration was 5.4 mo (0.7-30.3) with T-DXd and 4.6 mo (0.9-34.9) with RAM + PTX. Treatment-emergent adverse events (TEAEs) were reported in 244/244 (100%) vs 228/233 pts (97.9%) with T-DXd vs RAM + PTX, respectively; 68.0% vs 73.8% were grade (G) ≥3. Serious TEAEs with T-DXd vs RAM + PTX occurred in 41.0% vs 43.3% of pts; TEAEs associated with drug discontinuation occurred in 14.3% vs 17.2% of pts. Independently adjudicated drug-related interstitial lung disease/pneumonitis occurred in 34 pts (13.9%) with T-DXd (1 G3, 0 G4/5) vs 3 pts (1.3%) with RAM + PTX (2 G3, 1 G5).
At present, there are still many clinical trials of new anti-cancer technologies in China seeking patients. Consultation on new drugs and technologies, you can contact Beijing South Region Oncology Hospital International Department.
Phone Number:4008803716
Email:myimmnet@163.com
Post time: Jul-22-2025
