On March 1st, 2024, China National Medical Products Administration (NMPA) approved the launch of CAR-T cell product Zevorcabtagene AutoLeucel (Zevor-cel, CT053) for the treatment of adult patients with relapsed or refractory multiple myeloma who had progressed after at least 3 lines of treatment(using at least one proteasome inhibitor and immune modulator).
This listing is mainly based on an open-label, single-arm, multi-center phase II clinical trial (LUMMICAR STUDY 1, NCT03975907) in China, which achieved a 100% remission rate.
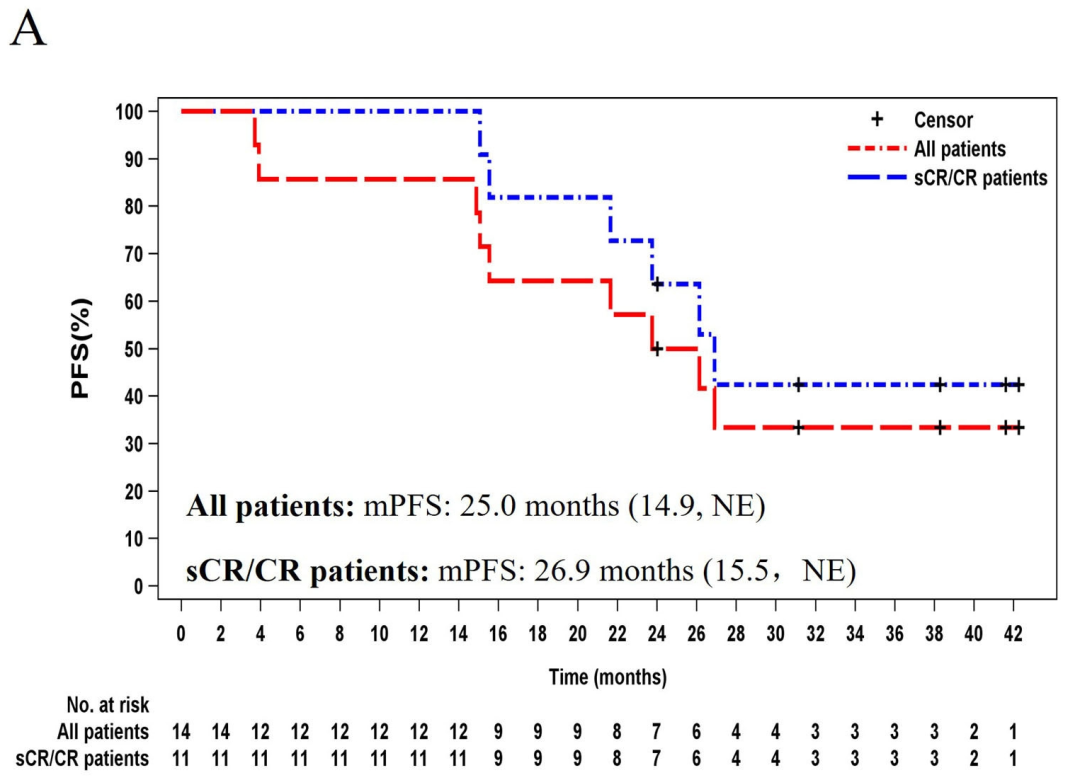
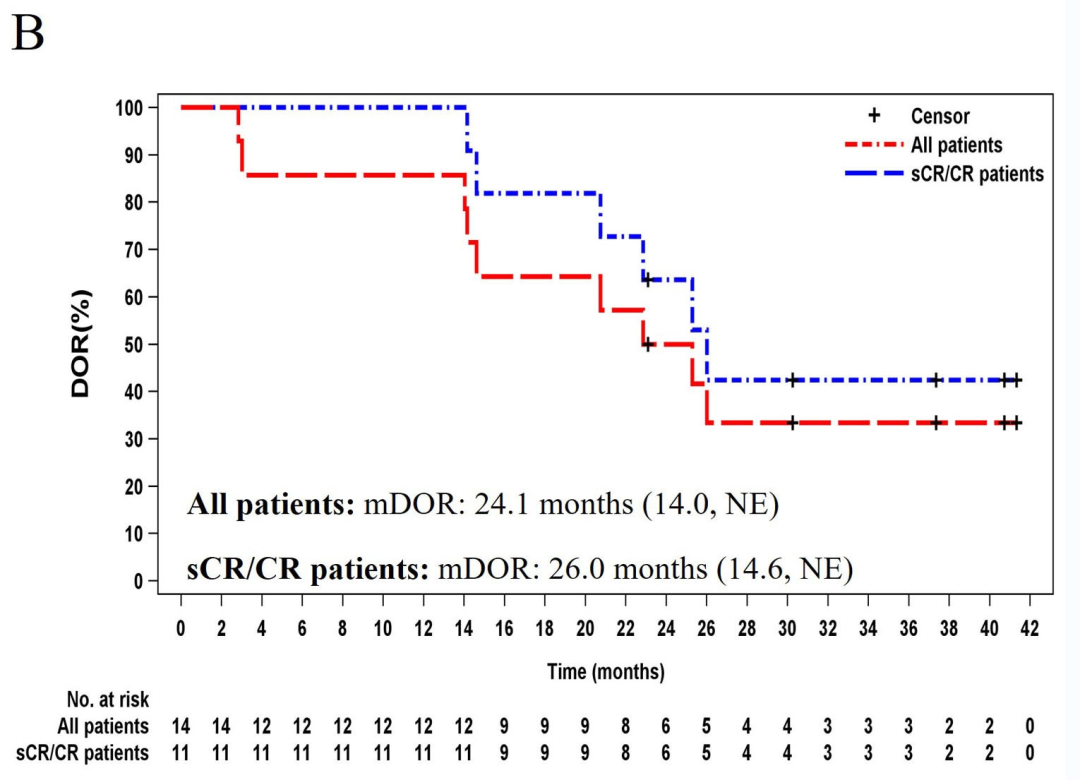
14 patients with a median age of 54 years (range:34-62), received a single infusion of zevor-cel.As of July 17, 2023, the median follow-up duration was 37.7 months (range:14.8-44.2 months). The overall response rate (ORR) was 100% (14/14) with 78.6% (11/14) patients achieving a complete response (CR) or a stringent complete response (sCR); minimal residual disease (MRD) negativity was attained in all patients achieving either a CR or sCR. The median duration of response (mDOR) was 24.1 months in all patients and 26.0 months in those achieving CR or sCR. The median progression-free survival (mPFS) was 25.0 months. A total of 7 (50%) patients were in remission lasting longer than 24 months. The median overall survival (OS) was not reached, and 92.9% (13/14) of patients were still alive at month 36.
Safety
Overall, the safety profile of zevor-cel was manageable. There were no ≥ Grade 3 cytokine release syndrome (CRS) events. There were no immune effector cell-associated neurotoxicity syndrome (ICANS) events of any grade. Three treatment-related Grade 3 infections were observed. Three patients experienced serious adverse events (SAE) including 2 patients who had treatment-related SAEs which were pulmonary infection and tumor lysis syndrome. There were overall 2 deaths on the study; neither was related to zevor-cel.
Conclusion
zevor-cel demonstrated an encouraging safety profile with deep and durable responses consistent with the initial results.
At present, there are many other CAR-T clinical trials in China, and they are looking for patients. For consultation on new drugs and technologies, you can contact Beijing South Region Oncology Hospital International Department: 4008803716.
References
2.http://sh.news.cn/20240314/2913c85a84be4fbf9345efbd48006971/c.html
3.http://myimm.net/mianyizhiliao/1413.html
4.https://www.nmpa.gov.cn/zwfw/sdxx/sdxxyp/yppjfb/20240301143753188.html
Post time: Oct-31-2024